Sign up for daily news updates from CleanTechnica on email. Or follow us on Google News!
Water, water everywhere and now we may have drops to drink
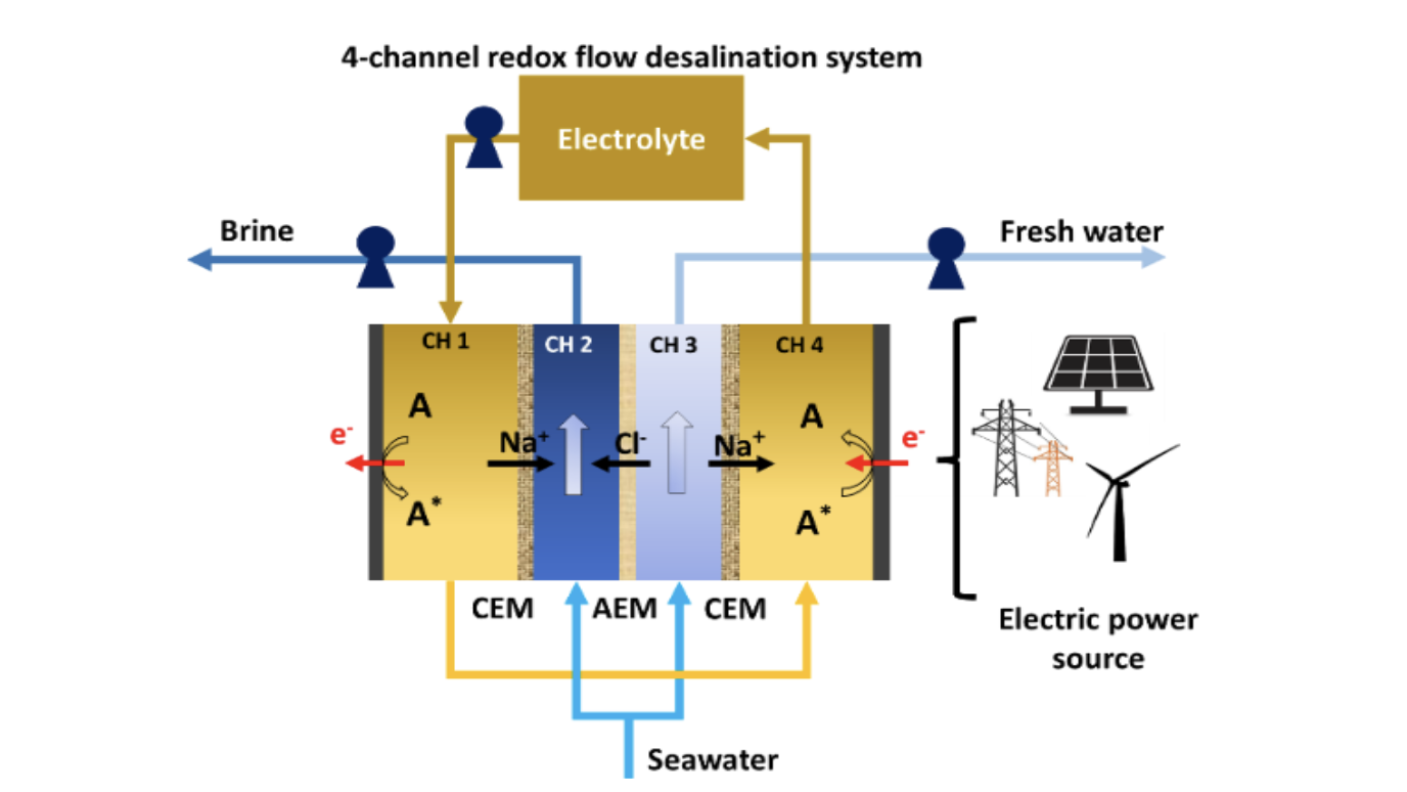
Schematic for a four-channel RFD in single-pass mode with an A/A*, representing electrochemical reactions of redox species dissolved in conducting salts solutions, and channels separated by cation-exchange membrane (CEM) and anion-exchange membrane (AEM).
Researchers at NYU Tandon School of Engineering achieved a major breakthrough in Redox Flow Desalination (RFD), an emerging electrochemical technique that can turn seawater into potable drinking water and also store affordable renewable energy.
In a paper published in Cell Reports Physical Science, the NYU Tandon team led by Dr. André Taylor, professor of chemical and biomolecular engineering and director of DC-MUSE (Decarbonizing Chemical Manufacturing Using Sustainable Electrification), increased the RFD system’s salt removal rate by approximately 20 percent while lowering its energy demand by optimizing fluid flow rates.
RFD offers multiple benefits. These systems provide a scalable and flexible approach to energy storage, enabling the efficient utilization of intermittent renewable energy sources such as solar and wind. RFD also promises an entirely new solution to the global water crisis.
“By seamlessly integrating energy storage and desalination, our vision is to create a sustainable and efficient solution that not only meets the growing demand for freshwater but also champions environmental conservation and renewable energy integration,” said Taylor.
RFD can both reduce reliance on conventional power grids and also foster the transition towards a carbon-neutral and eco-friendly water desalination process. Furthermore, the integration of redox flow batteries with desalination technologies enhances system efficiency and reliability.
The inherent ability of redox flow batteries to store excess energy during periods of abundance and discharge it during peak demand aligns seamlessly with the fluctuating energy requirements of desalination processes.
“The success of this project is attributed to the ingenuity and perseverance of Stephen Akwei Maclean, the paper’s first author and a NYU Tandon Ph.D. candidate in chemical and biomolecular engineering,” said Taylor. “He demonstrated exceptional skill by designing the system architecture using advanced 3D printing technology available at the NYU Maker Space.”
The intricacies of the system involve the division of incoming seawater into two streams: the salinating stream (Image above, CH 2) and the desalinating stream (Image above, CH 3). Two additional channels house the electrolyte and redox molecule (Image above, A). These channels are effectively separated by either a cation exchange membrane (CEM) or an anion exchange membrane (AEM).
In CH 4, electrons are supplied from the cathode to the redox molecule, extracting Na+ that diffuses from CH 3. The redox molecule and Na+ are then transported to CH 4, where electrons are supplied to the anode from the redox molecules, and Na+ is allowed to diffuse into CH 2. Under this overall potential, Cl- ions move from CH 3 through the AEM to CH 2, forming the concentrated brine stream. Consequently, CH 3 generates the freshwater stream.
“We can control the incoming seawater residence time to produce drinkable water by operating the system in a single pass or batch mode,” said Maclean.
In the reverse operation, where the brine and freshwater are mixed, the stored chemical energy can be converted into renewable electricity. In essence, RFD systems can serve as a unique form of “battery,” capturing excess energy stored from solar and wind sources.
This stored energy can be released on demand, providing a versatile and sustainable supplement to other electricity sources when needed. The dual functionality of the RFD system showcases its potential not only in desalination but also as an innovative contributor to renewable energy solutions.
While further research is warranted, the findings from the NYU Tandon team signal a promising avenue towards a more cost-effective RFD process – a critical advancement in the global quest for increased potable water. As climate change and population growth intensify, more regions grapple with water shortages, underscoring the significance of innovative and efficient desalination methods.
This research aligns seamlessly with the mission of DC-MUSE (Decarbonizing Chemical Manufacturing Using Sustainable Electrification), a collaborative initiative established at NYU Tandon. DC-MUSE is committed to advancing research activities that diminish the environmental impact of chemical processes through the utilization of renewable energy. The current study builds upon Taylor’s extensive body of work in renewable energy, with a recent emphasis on storing sustainably produced energy for utilization during off-peak hours.
In addition to Taylor and Maclean, the dedicated team of NYU Tandon researchers contributing to this study includes Syed Raza, Hang Wang, Chiamaka Igbomezie, Jamin Liu, Nathan Makowski, Yuanyuan Ma, Yaxin Shen, and Jason A. Röhrl. Collaborating across borders, Guo-Ming Weng from Shanghai Jiao Tong University in China also played a crucial role as a team member.
An exceptional milestone, this publication marks the 100th from Taylor’s Transformative Materials & Devices Lab. Originally established at Yale University in 2008 and subsequently relocated to NYU Tandon in 2018, the lab focuses on the development of innovative materials and devices for energy conversion and storage, reflecting Taylor’s enduring commitment to transformative research in the field.
Courtesy of NYU Tandon School of Engineering.
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Latest CleanTechnica TV Video
I don’t like paywalls. You don’t like paywalls. Who likes paywalls? Here at CleanTechnica, we implemented a limited paywall for a while, but it always felt wrong — and it was always tough to decide what we should put behind there. In theory, your most exclusive and best content goes behind a paywall. But then fewer people read it!! So, we’ve decided to completely nix paywalls here at CleanTechnica. But…
Thank you!
CleanTechnica uses affiliate links. See our policy here.