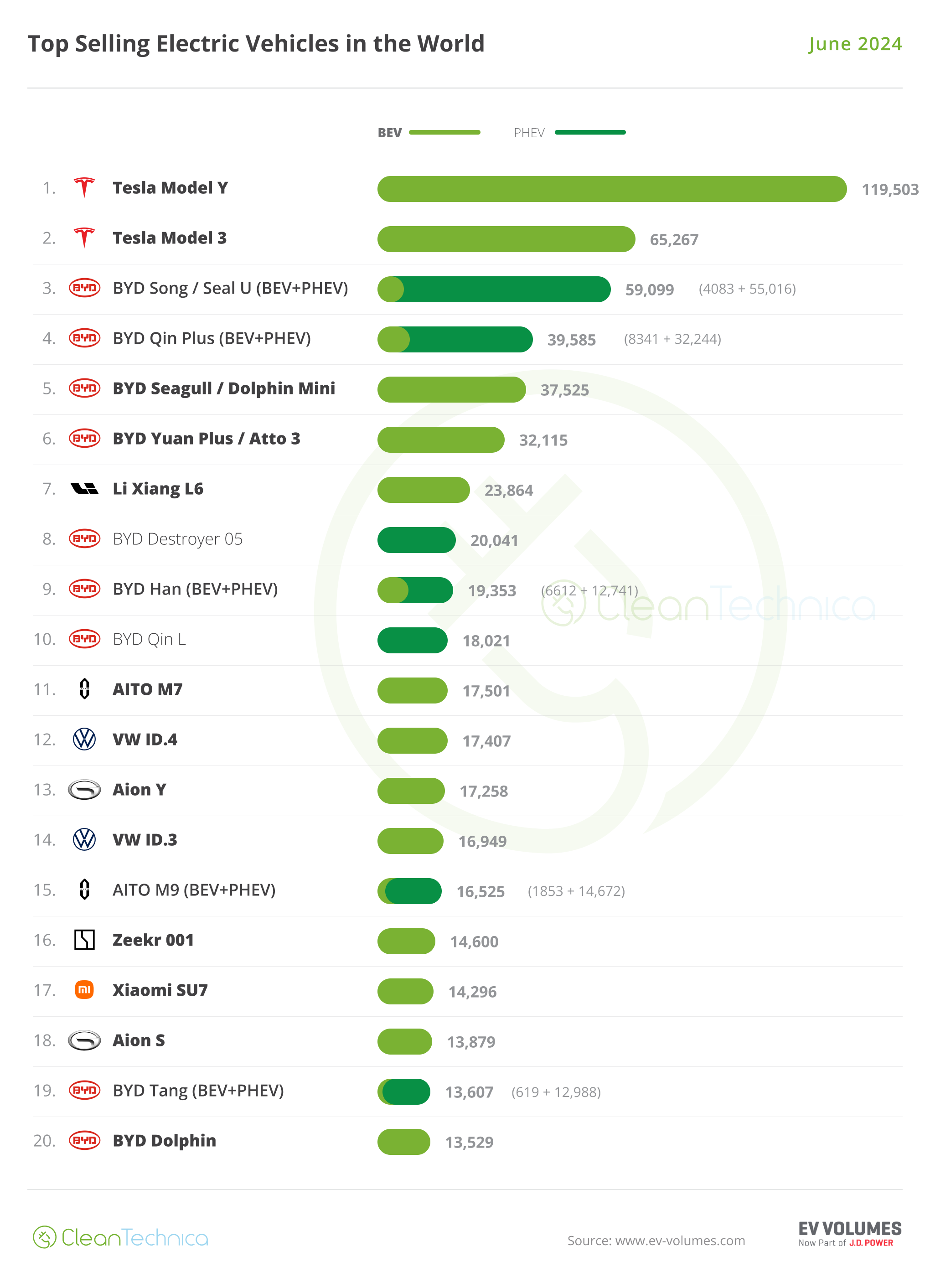
The global biopharmaceutical industry is coming to grips with an uncomfortable reality: after adjusting for the one-off returns generated by COVID-19 vaccines and therapeutics, R&D productivity has been anemic over the past decade, barely recouping the full value of capital invested. This has been the case whether it’s pipeline attrition rates, development timelines, or the costs of clinical trials. And productivity is well below what it was during the glory days of the late 1990s. Of course, there have been bright spots, but the industry has largely failed to make systemic gains (Exhibit 1).

Indeed, a more granular look at the underlying factors of R&D performance reveals the main culprits of this trend: slumping probabilities of success in clinical trials and rising costs of bringing new molecular entities (NMEs) to market (see sidebar, “The R&D productivity equation: Underlying factors of R&D performance”).
Moreover, in our analysis of the top 15 companies in the industry, there does not seem to be a strong correlation between R&D spend and NME value creation (Exhibit 2). Instead, we see that their R&D performance has largely been propelled by “blockbuster” medicines (NMEs that achieve over $1 billion in peak-year sales); these assets have buttressed overall productivity in the past decade (Exhibit 3).


Stagnant productivity is not a new problem, but it increasingly feels entrenched. The outlook for reimbursement erodes in major markets (especially in the United States as the Inflation Reduction Act takes effect), the impact of patent expirations for major franchises is expected to be significant (propelled by increasing biosimilar penetration), and pipeline competition intensifies (with increasing herding in high-potential disease areas and targets ).
To be sure, R&D productivity is only one way to measure biopharma success, and other measures are much more encouraging. From a shareholder perspective, biopharma has achieved admirable returns (roughly 9 percent on an annualized basis over the past decade). From a scientific perspective, the frontier of possibilities opened by novel modalities (today, 22 percent of the industry’s clinical pipeline is novel therapeutics) and the vast powers of AI are nothing short of breathtaking. And what’s most important, from a patient’s perspective, is the industry has provided numerous life-altering therapies across a wide range of diseases.
A recipe for boosting R&D performance
But productivity still matters. Over the past year, we have spoken with hundreds of R&D leaders who are determined to build on this innovation potential, crack the R&D productivity conundrum, and provide still more medicines that matter to patients. Based on those dialogues and our experience, we propose in this article a “recipe” of eight essential ingredients for sustained R&D success (Exhibit 4):
- charting the path to patients: effective asset and program strategies
- picking winning medicines: investor-like portfolio strategy optimized for risk and reward
- working simply and smartly: simplified, automated, and digitized core processes
- strengthening the backbone: enabling the R&D system (decision making, footprint, and organization)
- innovating with the external ecosystem: skills to identify and secure external assets
- bringing the future forward: next-generation data, analytics, and technology
- putting people at the center: a distinctive talent model
- partnering for joint success: streamlined vendor partnerships

None of these ingredients are surprising on their own; however, we’ve observed that meaningful productivity and performance gains can be achieved when they are tackled together, at scale, and at pace. Detailing this recipe and how to apply it to outperform is the focus of our ongoing research on biopharma R&D.
In upcoming publications, we will explore each of these ingredients in detail and describe the practices that distinguish industry leaders from laggards. For now, we offer this article as a high-level concept note that sets out what we believe are the eight essentials of successful, value-creating R&D, based on our research and discussions.
Effective asset and program strategies
Companies are gradually shaping a value-maximizing asset strategy (often starting as early as candidate selection) that fully explores line extensions and indication expansions to generate a future blockbuster drug. This requires actors to take on significant investment risk earlier, as they pursue parallel, pivotal, and enabling clinical programs to explore the full potential of an asset. This, in turn, is inducing companies to include insights powered by AI and machine learning (AI/ML) (for example, those informed by in silico disease and safety models) in decision making to derisk such investments.
Investor-like portfolio strategy optimized for risk and reward
Practically speaking, a “blockbuster seeking” portfolio strategy directs 80 percent or more of R&D investment to a subset of assets that have the potential to yield at least $3 billion in peak-year sales (it costs as much to develop a single successful NME) and uses the company’s distinctive R&D capabilities (for example, in disease biology understanding, relevant modalities, development expertise, or market knowledge and relationships). Shaping such a portfolio requires leadership teams to make investor-like trade-offs on a “live” basis (versus the classic annual review), informed by internal and external data (real-world evidence and randomized clinical trial data sets) readouts, regulatory guidance, and competitive developments and enabled by scenario-based simulations (such as efficient frontier modeling).
Simplified, automated, and digitized core processes
Process simplification, and automation, along the critical path has gradually come into focus in recent years as the industry races to get medicines to patients faster. Examples of value chain steps where we have observed material accelerations include preclinical candidate nomination to the first subject in (in which we have seen accelerations from 21 to 26 months to 12 to 15 months following optimization), enrollment (20 percent acceleration observed), and submissions (in which actors are increasingly delivering less than eight weeks from database lock to submission). Achieving these accelerations requires both embedding novel automation and technological solutions and absolute operational clarity on the critical path—and the accountabilities for each process step (often across functional silos) to sustainably boost performance.
Enabling the R&D system (decision making, footprint, and organization)
Operating models are evolving rapidly to provide fewer high-value assets, which will require higher-risk investment decisions early in the life cycle. Specifically, three important investment decisions are increasingly in focus: (1) early alignment on the “minimum viable” target product profile for an asset, (2) commitment to the end-to-end asset strategy (and “path to patient,” including staging of clinical activities), and (3) prioritization of the portfolio. Each of them requires absolute clarity on decision rights, as well as the insights (which, more and more often, are analytically powered) to inform the decision and the pace at which it should be made (to minimize periods of indecision). These shifts in decision rights are reshaping organizational designs, role profiles, and capability investments to ensure that decisions are informed by the right insights to maximize asset value and that the right level of talent is engaging at the right time.
Skills to identify and secure external assets
As the competition for externally sourced future-blockbuster assets intensifies across the industry, with the value of the top ten M&A deals in biopharma in 2023 surpassing those of the previous three years, R&D functions are playing an increasingly critical role in these portfolio-shaping transactions. R&D, for example, is closely collaborating with business development to apply AI/ML techniques for identifying promising NME candidates aligned with its disease area and modality priorities. Additionally, these functions are developing comprehensive plans for asset strategy and evidence generation to become the “partner of choice.” And once a transaction has closed, they move quickly to build and scale new capabilities or investigator relationships to maximize the potential of particularly late-stage transactions, which require significant cross-functional agility and investment.
Beyond this transaction-focused approach, companies are shaping their external ecosystems to secure preferential access to the latest early-stage, differentiated, and emerging scientific substrates, as well as a broad range of mechanisms, including incubators, venture capital–like corporate funds, capability sharing, and scientific community networks.
Next-generation data, analytics, and technology
The disruptive potential for data, analytics, and technology to unlock R&D productivity is clear; however, we have not yet observed actors that fully realize this potential across the end-to-end R&D value chain. There are foundational opportunities for applying automation (including generative AI) to core business processes, such as protocol writing, project and site management, data management, and content authoring. Such uses can accelerate activities, limit operational variation, and reduce costs. As these new technologies are embedded, they could be integrated into a unified data platform with access to internal and external data sets to enable predictive analytics that can unlock further productivity gains. Examples include but are not limited to predictive target identification (using knowledge graphs of [multi]omics, randomized control trials, and real-world evidence data), lead design simulations, in silico preclinical models, and trial design simulators. Ultimately, the goal of applying such analytics is to create learning loops whereby experimental data is fed back to power improved analytical design; that, in turn, can propel improvements in success rates and cycle time acceleration. We have observed, for instance, companies creating such learning loops when moving molecules from lead identification to investigational-new-drug submission nine months faster, while also improving probability of success—specifically a fourfold improvement in leading indicators.
A distinctive talent model
The battle for capabilities and talent is intensifying as actors herd in blockbuster-yielding disease areas and focus on achieving R&D productivity improvements. This is causing a return to strategic workforce planning focused on both reimagined “old skills” (such as those possessed by bench scientists, asset team leaders for priority assets, clinical scientists, biostatisticians, regulatory strategists, and innovation scouts) and “new skills” (such as those possessed by new platform technology experts, experts in areas like cell and gene therapy, AI/ML-capable data scientists, and performance transformation leads). Talent transformations may involve upgrading the talent base by replacing those who work on deprioritized diseases and technologies and upgrading associated automated activities with best-in-industry models suitable for the current priorities. New hires can be offered attractive career paths beyond their starting roles. Any remaining talent gaps can be closed through partnerships (particularly for AI/ML capabilities), with a clear path to embed these capabilities internally.
Streamlined vendor partnerships
Pharmaceutical companies are increasingly reliant on vendors (which they share with their peers) to help them achieve their industry performance goals. Hence, it’s critical that companies redefine their relationships with these partners. Across the industry, as actors race to secure new technical capabilities and enter new disease areas, there is an increased focus on streamlining access to cost-effective, nondifferentiated capacity while simultaneously identifying “on demand” capabilities needed to scale; defragmenting across this vendor base accordingly; aligning incentives in contracting; developing joint working models; and applying technical solutions that allow both partners to outperform.
Applying the recipe in practice
R&D engines are complex. Achieving a step change in performance while also growing a portfolio of potential blockbuster medicines requires a continuous transformation muscle—few companies have it, but those that do are able to reap tangible, at-scale performance improvements. Like all sustained transformations, effective R&D performance transformations typically involve the following elements:
- detailed execution plan designed to achieve “full potential” performance improvement (informed by benchmarks or use cases where possible)
- aligned metrics that indicate near-term impact (versus progress); delivery of initiatives (“quarterly value releases”)
- dedicated initiative-scaling engine to ensure test cases (for example, at the asset level) are adopted across R&D as soon as value is demonstrated
- focused change management efforts to enable the required behavioral shifts, cascading communications, objective setting and rewards, and leadership role modeling
Pharma companies across the industry are beginning to make significant investment shifts in response to mounting external pressures. Pipelines are being refocused, material capability investments are being made, and new partnerships are being forged—all to enable a gear change in R&D engine performance. There is excitement across the industry that, against this backdrop, the decades-old problem of R&D productivity may finally be cracked and a growing recognition that those that fail to tackle this head-on may soon be left behind.