
Sign up for daily news updates from CleanTechnica on email. Or follow us on Google News!
Lithium extraction from alternate sources is crucial for the renewable energy transition and resource independence in countries such as the United States. Researchers continue to discover methods to obtain efficient and ecologically sound lithium. In partnership with other organizations, researchers at George Washington University have created a novel technique for directly extracting and purifying lithium from geothermal brines, which can be utilized to create batteries for electric vehicles. Because, after all, environmentally friendly lithium batteries are the petrol tanks of the wiser traveler.
This method uses a unique substance that selectively extracts lithium ions from the brine, avoiding harsh chemicals that could harm the environment. After being captured, the lithium is transformed into lithium hydroxide, which is the grade appropriate for batteries used in electric vehicles, and subsequently lithium chloride. The approach might manufacture battery-grade lithium at a competitive cost, according to the team’s additional economic research.
According to the experts, present lithium supplies, such as hard rock mining and salt flats, are inadequate to meet future world demand. This study proposes an environmentally beneficial alternative that uses geothermal brines, specifically from sources such as the Salton Sea in California. The researchers’ technology will be tested at scale in the next few years.
The study, “Electro-driven direct lithium extraction from geothermal brines to generate battery-grade lithium hydroxide,” was published in Nature Communications.
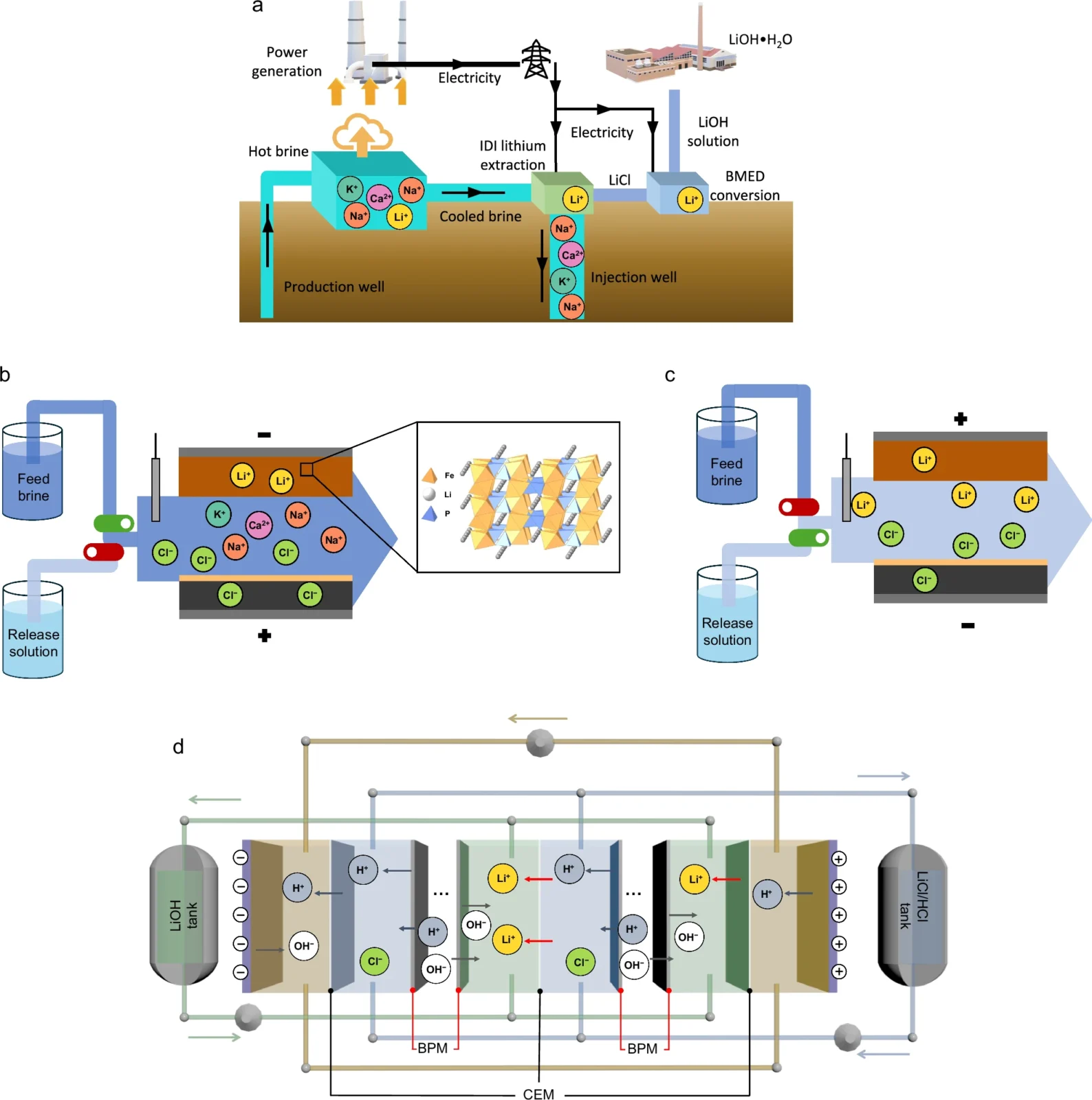
Schematic demonstration of electro-driven lithium extraction and lithium hydroxide conversion processes.
Lead researcher Xitong Liu is a professor in the Department of Civil and Environmental Engineering at the George Washington University School of Engineering and Applied Science.
All Researchers: Lingchen Kong, Gangbin Yan, Kejia Hu, Yongchang Yu, Nicole Conte, Kevin R. Mckenzie Jr, Michael J. Wagner, Stephen G. Boyes, Hanning Chen, Chong Liu, and Xitong Liu.
Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Sign up for our daily newsletter for 15 new cleantech stories a day. Or sign up for our weekly one if daily is too frequent.
CleanTechnica uses affiliate links. See our policy here.
CleanTechnica’s Comment Policy


