
Pouring flecks of rust into water usually makes it dirtier. But researchers have developed special iron oxide nanoparticles they call “smart rust” that actually makes it cleaner. Smart rust can attract many substances, including oil, nano- and microplastics, as well as the herbicide glyphosate, depending on the particles’ coating. And because the nanoparticles are magnetic, they can easily be removed from water with a magnet along with the pollutants. Now, the team is reporting that they’ve tweaked the particles to trap estrogen hormones that are potentially harmful to aquatic life.
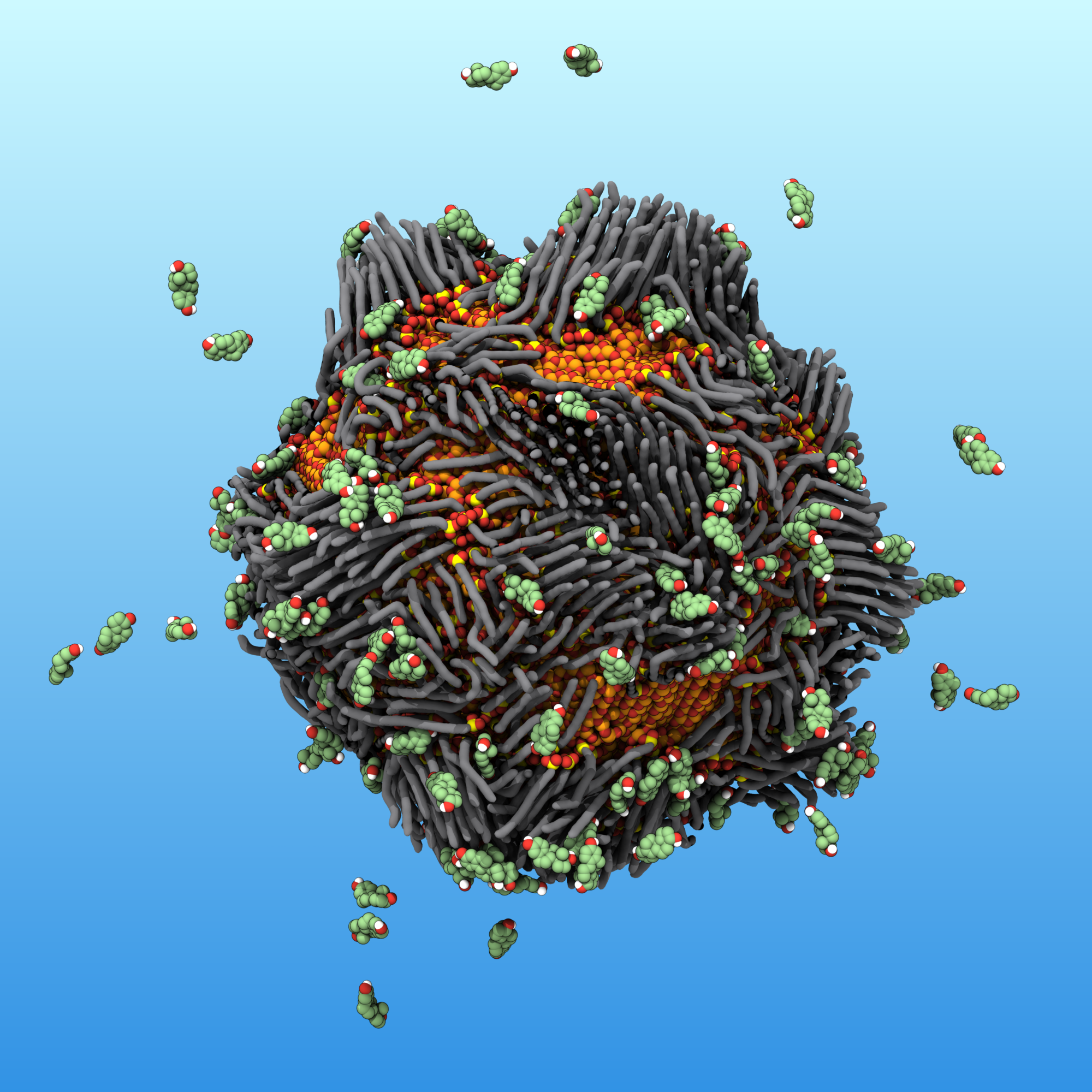
In this illustration, a “smart rust” nanoparticle attracts and traps estrogen molecules, which are represented by the floating objects. Dr. Dustin Vivod and Prof. Dr. Dirk Zahn, Computer Chemistry Center (CCC), Friedrich-Alexander-Universität Erlangen-Nürnberg
The researchers will present their results today at the fall meeting of the American Chemical Society (ACS). ACS Fall 2023 is a hybrid meeting being held virtually and in-person Aug. 13–17, and features about 12,000 presentations on a wide range of science topics.
“Our ‘smart rust’ is cheap, nontoxic and recyclable,” says Marcus Halik, Ph.D., the project’s principal investigator. “And we have demonstrated its use for all kinds of contaminants, showing the potential for this technique to improve water treatment dramatically.”
For many years, Halik’s research team has been investigating environmentally friendly ways to remove pollutants from water. The base materials they use are iron oxide nanoparticles in a superparamagnetic form, which means they are drawn to magnets, but not to each other, so the particles don’t clump.
To make them “smart,” the team developed a technique to attach phosphonic acid molecules onto the nanometer-sized spheres. “After we add a layer of the molecules to the iron oxide cores, they look like hairs sticking out of these particles’ surfaces,” says Halik, who is at Friedrich-Alexander-Universität Erlangen-Nürnberg. Then, by changing what is bound to the other side of the phosphonic acids, the researchers can tune the properties of the nanoparticles’ surfaces to strongly adsorb different types of pollutants.
Early versions of smart rust trapped crude oil from water collected from the Mediterranean Sea and glyphosate from pond water collected near the researchers’ university. Additionally, the team demonstrated that smart rust could remove nano- and microplastics added to lab and river water samples.
So far, the team has targeted pollutants present in mostly large amounts. Lukas Müller, a graduate student who’s presenting new work at the meeting, wanted to know if he could modify the rust nanoparticles to attract trace contaminants, such as hormones. When some of our body’s hormones are excreted, they are flushed into wastewater and eventually enter waterways. Natural and synthetic estrogens are one such group of hormones, and the main sources of these contaminants include waste from humans and livestock. The amounts of estrogens are very low in the environment, says Müller, so they are difficult to remove. Yet even these levels have been shown to affect the metabolism and reproduction of some plants and animals, although the effects of low levels of these compounds on humans over long periods aren’t fully known.
“I started with the most common estrogen, estradiol, and then four other derivatives that share similar molecular structures,” says Müller. Estrogen molecules have a bulky steroid body and parts with slight negative charges. To exploit both characteristics, he coated iron oxide nanoparticles with two sets of compounds: one that’s long and another that’s positively charged. The two molecules organized themselves on the nanoparticles’ surface, and the researchers hypothesize that together, they build many billions of “pockets” that draw in the estradiol and trap it in place.
Because these pockets are invisible to the naked eye, Müller has been using high-tech instruments to verify that these estrogen-trapping pockets exist. Preliminary results show efficient extraction of the hormones from lab samples, but the researchers need to look at additional experiments from solid-state nuclear magnetic resonance spectroscopy and small-angle neutron scattering to verify the pocket hypothesis. “We are trying to use different puzzle pieces to understand how the molecules actually assemble on the nanoparticles’ surface,” explains Müller.
In the future, the team will test these particles on real-world water samples and determine the number of times that they can be reused. Because each nanoparticle has a high surface area with lots of pockets, the researchers say that they should be able to remove estrogens from multiple water samples, thereby reducing the cost per cleaning. “By repeatedly recycling these particles, the material impact from this water treatment method could become very small,” concludes Halik.
The researchers acknowledge support and funding from the German Research Foundation, the German Federal Environmental Foundation and Friedrich-Alexander-Universität Erlangen-Nürnberg.
Courtesy of the American Chemical Society.
This research was presented at a meeting of the American Chemical Society
I don’t like paywalls. You don’t like paywalls. Who likes paywalls? Here at CleanTechnica, we implemented a limited paywall for a while, but it always felt wrong — and it was always tough to decide what we should put behind there. In theory, your most exclusive and best content goes behind a paywall. But then fewer people read it! We just don’t like paywalls, and so we’ve decided to ditch ours. Unfortunately, the media business is still a tough, cut-throat business with tiny margins. It’s a never-ending Olympic challenge to stay above water or even perhaps — gasp — grow. So …




