
A research team led by Gao Liu, a senior scientist in the Energy Technologies Area at Lawrence Berkeley Lab, published a paper in the journal Nature Energy recently in which they report on new technology that could lower the cost of lithium-ion batteries and extend their service life. Here’s the abstract:
Electrically conductive polymers have found increasing applications in energy conversion and storage devices. In the conventional design of conductive polymers, organic functionalities are introduced via bottom-up synthetic approaches to enhance specific properties by modification of the individual polymers. Unfortunately, the addition of functional groups leads to conflicting effects, limiting their scaled synthesis and broad applications.
Here we show a conductive polymer with simple primary building blocks that can be thermally processed to develop hierarchically ordered structures (HOS) with well-defined nanocrystalline morphologies. Our approach to constructing permanent HOS in conductive polymers leads to substantial enhancement of charge transport properties and mechanical robustness, which are critical for practical lithium-ion batteries.
Finally, we demonstrate that conductive polymers with HOS enable exceptional cycling performance of full cells with high-loading micron-size SiOx-based anodes, delivering areal capacities of more than 3.0 mAh cm−2 over 300 cycles and average Coulombic efficiency of >99.95%.
“The advance opens up a new approach to developing EV batteries that are more affordable and easy to manufacture,” Liu said. The big news here is that the so-called HOS-PFM coating conducts both electrons and ions at the same time, which ensures battery stability and high charge/discharge rates while enhancing battery life. The coating also shows promise as a battery adhesive that could extend the lifetime of a lithium-ion battery from an average of 10 years to about 15 years, he added.
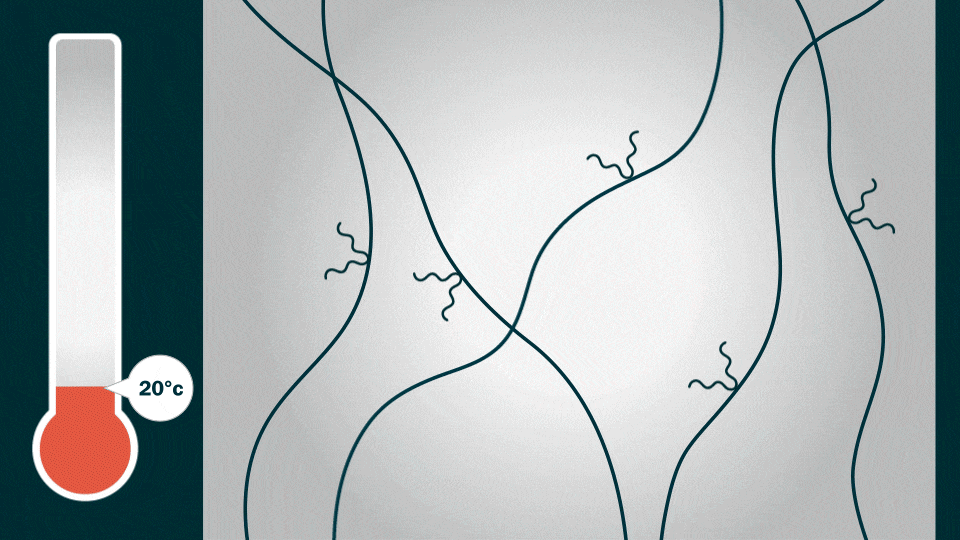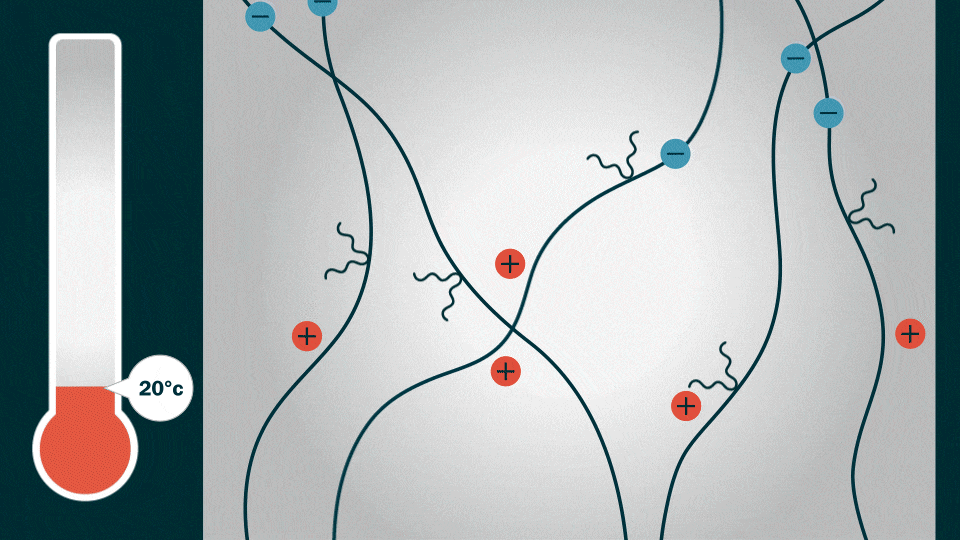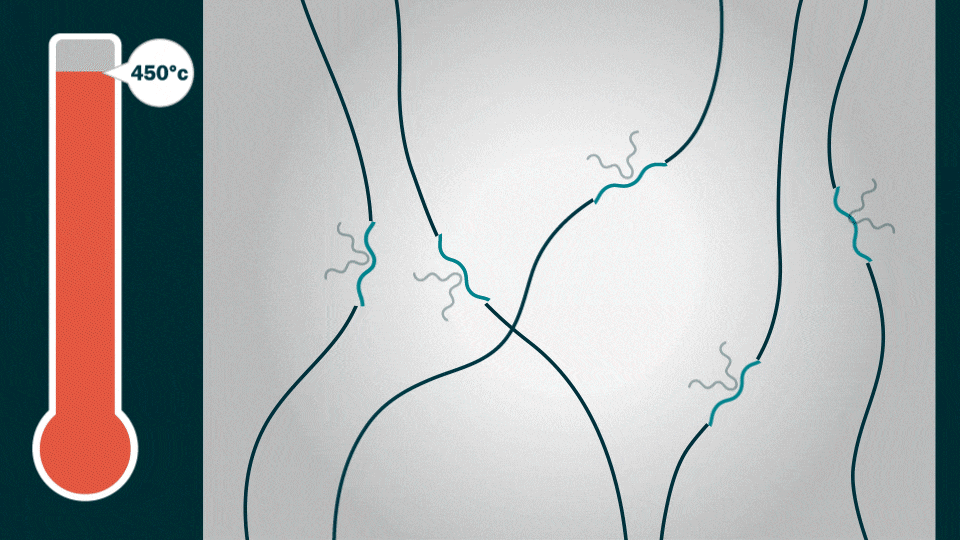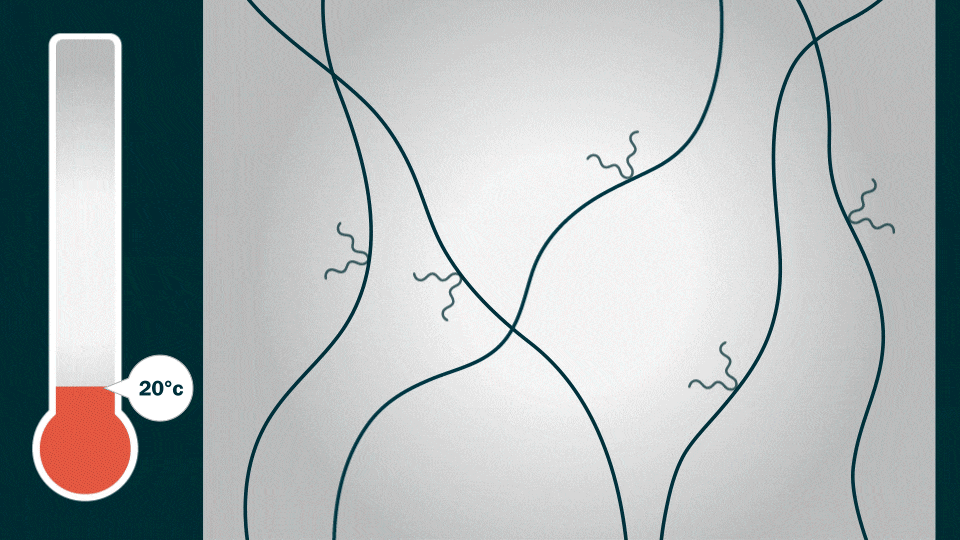
Credit: Jenny Nuss, Berkeley Lab
This is the caption to the graphic shown above from Berkeley Lab:
“Before heating: At room temperature (20 degrees Celsius), alkyl end-chains (black squiggly lines) on the PFM polymer chain limit the movement of lithium ions (red circles).
“When heated to about 450 degrees Celsius (842 degrees Fahrenheit), the alkyl end-chains melt away, creating vacant “sticky” sites (blue squiggly lines) that “grab” onto silicon or aluminum materials at the atomic level. PFM’s polymer chains then self-assemble into spaghetti-like strands called “hierarchically ordered structures” or HOS.
“Like an atomic expressway, the HOS-PFM strands allow lithium ions to hitch a ride with electrons (blue circles). These lithium ions and electrons move in synchronicity along the aligned conductive polymer chains.”
Better Batteries From Berkeley Lab
If you are following so far, read on. To demonstrate HOS-PFM’s superior conductive and adhesive properties, Liu and his team coated aluminum and silicon electrodes with HOS-PFM, and tested their performance in a lithium-ion battery setup. Silicon and aluminum are promising electrode materials for lithium-ion batteries because of their potentially high energy storage capacity and lightweight profiles. But these cheap and abundant materials quickly wear down after multiple charge/discharge cycles.
During experiments at the Advanced Light Source and the Molecular Foundry, which is part of the Lawrence Berkeley Lab, the researchers demonstrated that the HOS-PFM coating significantly prevents silicon- and aluminum-based electrodes from degrading during battery cycling while delivering high battery capacity over 300 cycles, a performance rate on par with today’s state of the art electrodes.
The results are impressive, Liu said, because silicon-based lithium-ion cells typically last for a limited number of charge/discharge cycles and calendar life. The researchers successfully demonstrated that the HOS-PFM coating significantly prevents aluminum-based electrodes from degrading during battery cycling while delivering high battery capacity over 300 cycles. “The advance opens up a new approach to developing EV batteries that are more affordable and easy to manufacture,” Gao said.
The HOS-PFM coating could allow the use of electrodes containing as much as 80% silicon. Such high silicon content could increase the energy density of lithium-ion batteries by at least 30%, Liu said. In addition, because silicon is cheaper than graphite — which is the standard material for electrodes today — cheaper batteries could significantly increase the availability of entry level electric vehicles, he added.

Now The Hard Work Begins
The team next plans to work with companies to scale up HOS-PFM for mass manufacturing. Regular readers are well aware that battery breakthroughs happen just about every day. CATL this week announced new chemistries that allow batteries to function better in cold temperatures. Toyota claims it will have solid-state batteries in production by 2026. Interest in sodium-ion batteries is sky high because they hold the promise of avoiding the use of lithium altogether.
The primary focus right now is less about the lifetime of batteries — the initial fears a decade ago that EV batteries would need to be replaced after two to three years have faded — than it is about costs and the environmental implications of mining the cobalt, lithium, manganese, and nickel needed to make them.
Oddly, nobody ever questions the environmental implications of extracting oil and methane gas from below the surface of the Earth or the consequences of pumping billions of tons of carbon dioxide into the atmosphere. No one ever acknowledges that cobalt is used in the refining of crude oil. Somehow it is perfectly OK for young children in the Democratic Republic of Congo to harvest cobalt from the Earth if it is being used to make gasoline or plastics, but not OK to do the same if it is used to make batteries. The hypocrisy of fossil fuel acolytes is never ending and simply breathtaking.
Nonetheless, the quest for batteries that have higher energy densities and lower costs is vital if the EV revolution is to fully succeed. Moving forward, Lawrence Berkeley Lab will need to share its technology with several battery manufacturers who will make prototype batteries using this new technology. Then those batteries will be supplied to several automakers who will use them to conduct real world testing.
If they measure up, if they perform as expected, and if they are less expensive than the batteries those manufacturers are currently using, then they may find their way into production vehicles. Typically, all those “ifs” mean commercial applications are about 5 years away, and that is the best case scenario.
The takeaway from all this is that better batteries are coming. The batteries of tomorrow will be more powerful, more durable, and cost less than anything available today. The real question is whether they will arrive in time to finally convince the world to ditch its fossil fuel habit.
I don’t like paywalls. You don’t like paywalls. Who likes paywalls? Here at CleanTechnica, we implemented a limited paywall for a while, but it always felt wrong — and it was always tough to decide what we should put behind there. In theory, your most exclusive and best content goes behind a paywall. But then fewer people read it! We just don’t like paywalls, and so we’ve decided to ditch ours. Unfortunately, the media business is still a tough, cut-throat business with tiny margins. It’s a never-ending Olympic challenge to stay above water or even perhaps — gasp — grow. So …



